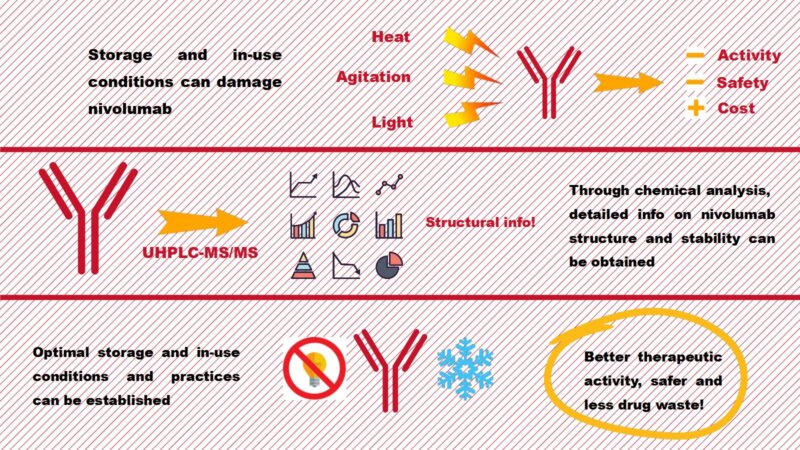
Quality control of drugs is an integral part of the pharmaceutical industry and is fundamental for their commercialization and correct administration in hospitals. Nivolumab, which is sold under the tradename Opdivo®, is a therapeutic antibody used for the treatment of cancer worldwide. It is currently one of the most expensive drugs on the market due to its high production costs. As a biopharmaceutical drug, it is highly vulnerable to various kinds of stress (thermal, mechanical, light exposure, etc.) to which it may be exposed during preparation for administration in hospitals. These stresses could trigger its degradation and potentially affect its therapeutic activity. Thus, a detailed study of the chemical structure of nivolumab is necessary to ensure the right conditions for its storage and use in hospitals. In this project, we develop a method for studying the structure of nivolumab. Using Ultra High-Performance Liquid Chromatography coupled to tandem Mass Spectrometry (UHPLC-MS/MS), a powerful analytical tool, we provide valuable information on the structure and stability of nivolumab, so as to help optimize its usage and safety profile in hospitals worldwide.
Keywords: Nivolumab (Opdivo®); therapeutic antibody; biopharmaceutical; structural characterization.
Directed by: Natalia África Navas Iglesias
Leave a Reply