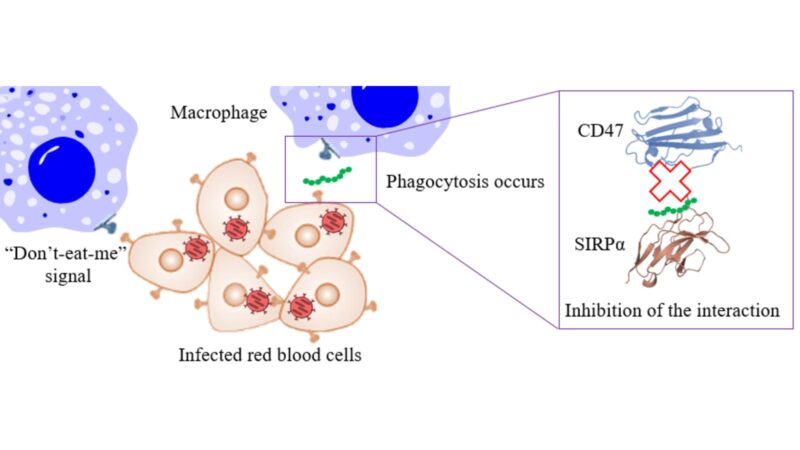
CD47 is a protein found on the surface of the cells of infectious agents, such as viruses and bacteria. Its interaction with the signal-regulatory protein alpha (SIRPα) of the cells in our immune system induces an antiphagocytic signal, thus preventing phagocytosis, the process by which the cells in our immune system trap infectious agents and eliminate them.
Certain tumours use this mechanism to escape the action of the immune system. The same mechanism is also used by pathogens, such as the SARS-CoV-2 coronavirus or Mycobacterium tuberculosis, which induce excessive expression of CD47 to overcome our body’s defence barriers easily. Since the activation of CD47 expression is not related to the specific interaction of the pathogen with our cells, it could be used as a therapeutic target for dealing with a wide variety of infectious agents. In fact, the use of compounds with anti-CD47 activity during viral infection has been shown to promote immunity and help clear the virus more quickly.
The main objective of this work is to discover new peptides (small proteins with less than 50 amino acids), which are resistant to the enzymes that break them down (proteases) and have high affinity for either CD47 or SIRPα, so as to inhibit the interaction between them.
The activity of these peptides will then be evaluated through what is known as affinity assays. The aim of these assays is to measure the degree of affinity of the peptides with these proteins and find out more about how they bind and interact with them.
Keywords: CD47; SIRPα; peptides; infections
Directed by: Macarena Sánchez Navarro