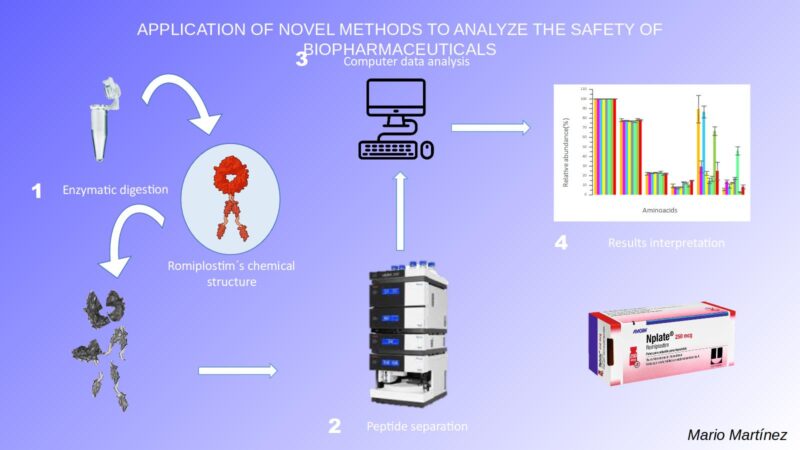
Biopharmaceuticals are pharmaceutical products manufactured from biological sources, such as cell cultures, animals, etc. Examples of biopharmaceuticals include vaccines and therapeutic proteins, among others. In this research, we present a study to evaluate the safety of romiplostim, the active ingredient of drug, used to increase the level of platelets in blood. Romiplostim is a protein and as such, is susceptible to changes in its structure. These changes can be caused, for example, by exposure to high temperatures or poor storage. This can inhibit the function of the drug or even produce undesirable reactions in the patient.
In this research, we analyze these structural modifications using a multi-attribute method, involving digestion of the romiplostim (cleavage of its structure into peptides), separation of the resulting peptides and detection by mass spectrometry. The drug is subjected to certain specific situations in the laboratory that might induce changes in its structure (for example, exposure to light). In this way, we evaluate the stability of its properties when subject to a range of different conditions, in order to assess and ensure its safe use.
Keywords: biopharmaceuticals; therapeutic proteins; romiplostim; multi-attribute method; safety
Directed by: Natalia Navas Iglesias
Leave a Reply